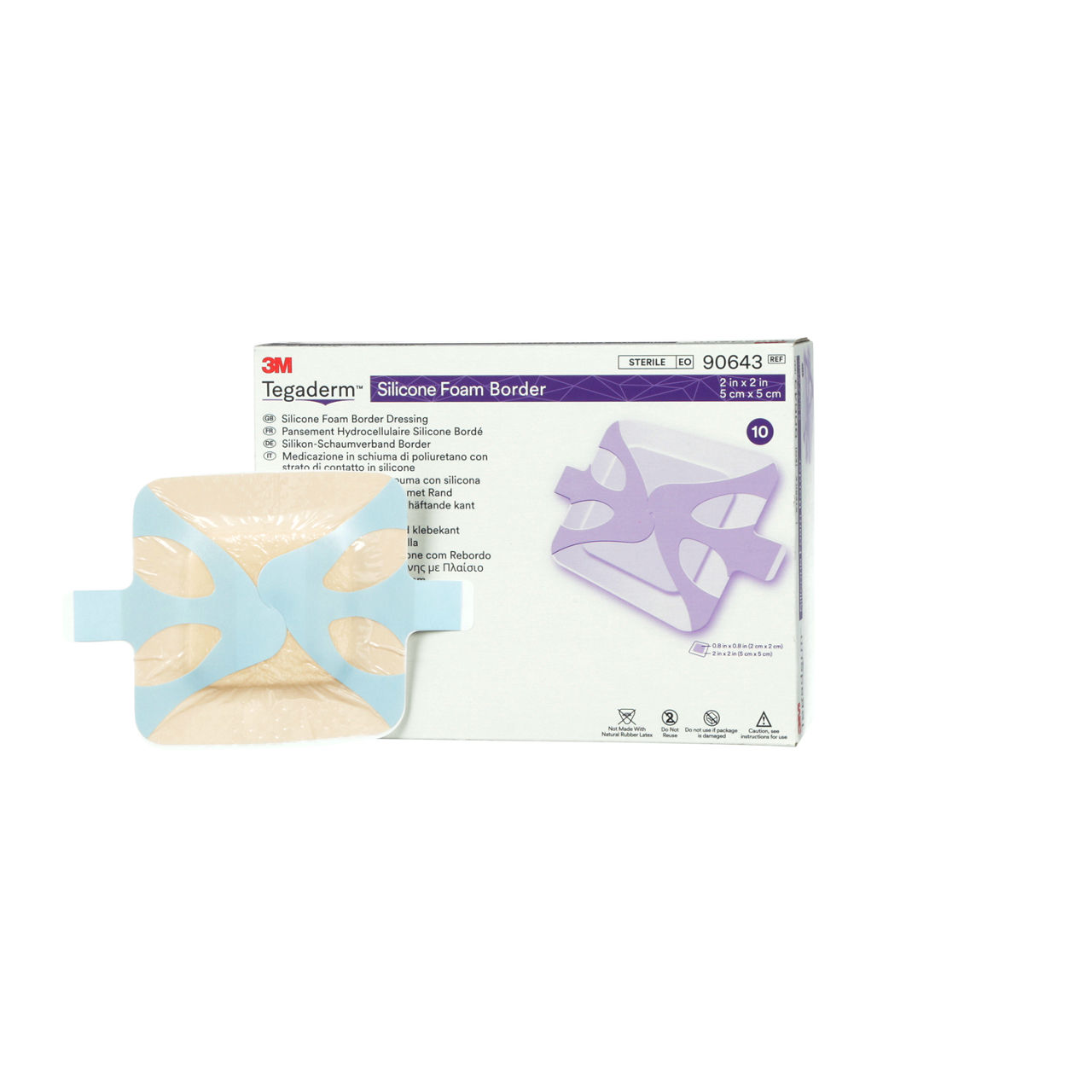
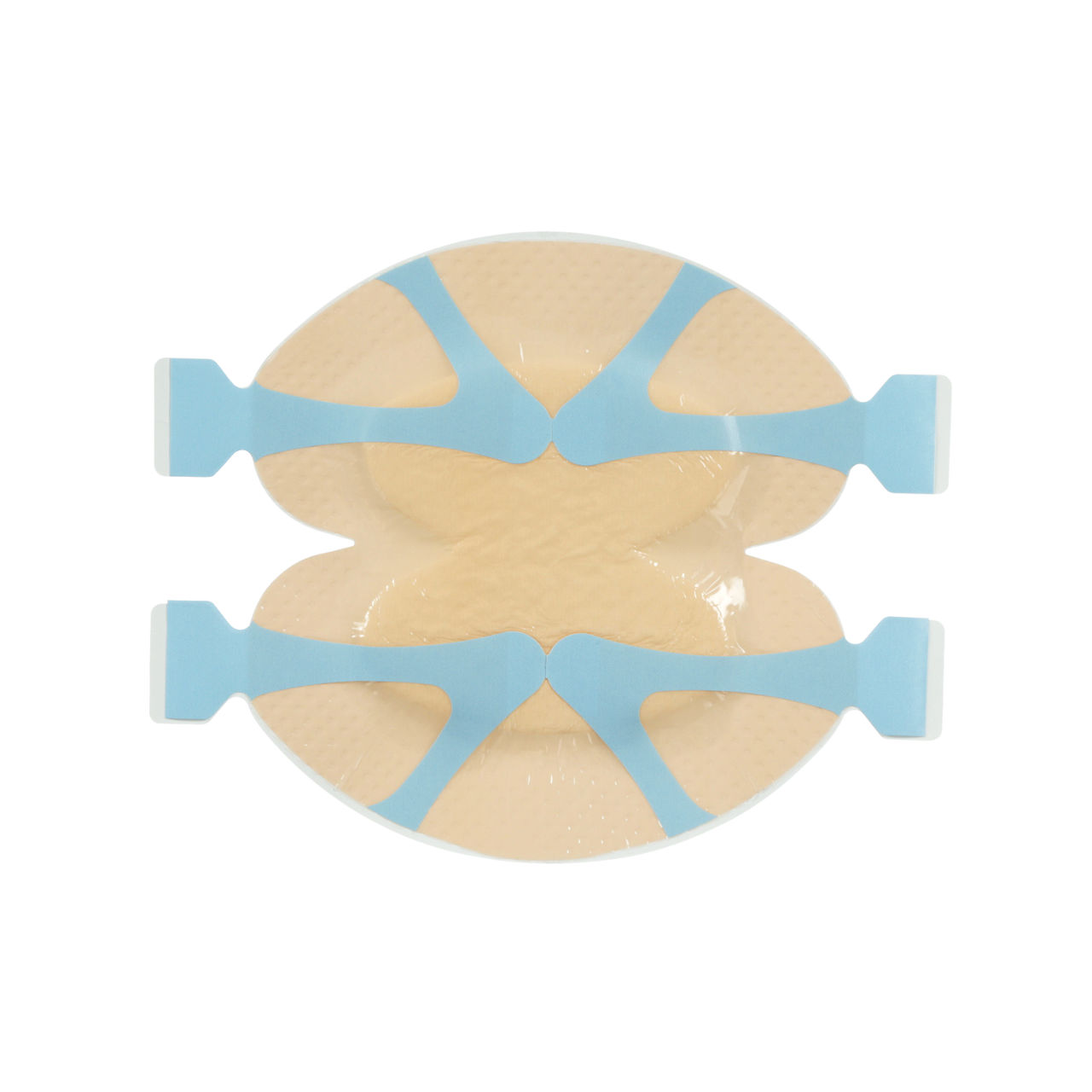
3M™ Tegaderm™ Silicone Border Foam Dressing
About the product
3M’s most advanced silicone foam dressing, the 3M™ Tegaderm™ Silicone Foam Border Dressing, takes on your toughest challenges impacting adhesion and wear time. It is suitable for use on fragile skin and with compression therapy, making it an appropriate choice for your wound management and pressure/ulcer injury1 prevention programs.
Product details
The 3M™ Tegaderm™ Silicone Foam Border Dressing stays in place with minimal lifting and rolling. It is an appropriate choice for your wound management and pressure injury prevention programs. Tegaderm Silicone Foam dressing adhesive is gentle even on fragile skin and does not cause damage to the wound, periwound or surrounding skin.
It can be used with compression therapy and is designed to manage varying levels of exudate, from low to high, such as pressure ulcers/injuries(1), venous leg ulcers, neuropathic ulcers, arterial ulcers, skin tears and surgical wounds.
- Advanced adhesive allows for “lift and check” interval monitoring
- Innovative spoke delivery system enables accurate, easy application with gloved hands for a more positive clinician experience
- Unique multi-layer design absorbs and evaporates moisture while adapting to changing levels of exudate to maintain an optimal wound healing environment
- Dressing is soft and conformable with a thin, low-profile edge to help minimise the rolling and lifting that can impact adhesion and wear time
- Adhesive film backing acts as a protective barrier to outside contamination, including bacteria and viruses* *In vitro testing shows that the transparent film provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
- Dressing is waterproof, so it may be worn during showering
- Interested in a non-silicone version of this foam dressing? View the 3M™ Tegaderm™ High Performance Foam Adhesive Dressing
- Film backing prevents strikethrough and protects against external bacteria and viruses*
Suggested Applications
- Wound management
- Pressure ulcer/injury prevention
- Tube/device cushioning or under compression
Legal
*4x4 and 6x6 dressings, based on In vivo studies EM-13977 and EM-13978. Solventum data on file.Product specifications
| Shape |
Shape
Square, Rectangular |
| OverallLengthImperial |
OverallLengthImperial
6.5 in |
| Size |
Size
Large |
| OverallWidthMetric |
OverallWidthMetric
5.08 cm |
| categoryName |
categoryName
Foam Dressings |
| Wound Dressing Width (Imperial) |
Wound Dressing Width (Imperial)
8.661 in |
| AdhesiveType |
AdhesiveType
Silicone |
| Brand |
Brand
Tegaderm™ |
| WoundDressingLengthImperial |
WoundDressingLengthImperial
8.661 in |
| Overall Length (Imperial) |
Overall Length (Imperial)
2 in, 8.75 in |
| Overall Width (Metric) |
Overall Width (Metric)
18.5 cm, 16.5 cm |
| Overall Length (Metric) |
Overall Length (Metric)
5.08 cm, 22.225 cm, 16.5 cm |
| Category name |
Category name
Foam Dressings |
| Overall Width (Imperial) |
Overall Width (Imperial)
2 in, 7.28 in, 6.5 in |
| Wound Dressing Length (Metric) |
Wound Dressing Length (Metric)
22 cm |
| Border |
Border
true |
| Wound Dressing Width (Metric) |
Wound Dressing Width (Metric)
22 cm |
| Adhesive Type |
Adhesive Type
Silicone |